Carl Zeiss Meditec is introducing the ZEISS Cataract Suite featuring the new CALLISTO eye® OR management system, which recently received US FDA 510k clearance, at the 2013 American Society of Cataract and Refractive Surgery (ASCRS) Symposium and Congress in San Francisco.
 ZEISS Cataract Suite (Photo: Business Wire)
ZEISS Cataract Suite (Photo: Business Wire)
The company also announces the US release of the FORUM® Viewer App which provides doctors with mobile access to the broad range of ZEISS and third-party diagnostic images and reports. The Medical Monitor of the VisuMax® IDE study, Jon Disher, MD, will present an update during the ASCRS plenary session on the US clinical trial for the VisuMax ReLEx® smile procedure for the correction of spherical myopia.
Introduction of the ZEISS Cataract Suite featuring CALLISTO eye
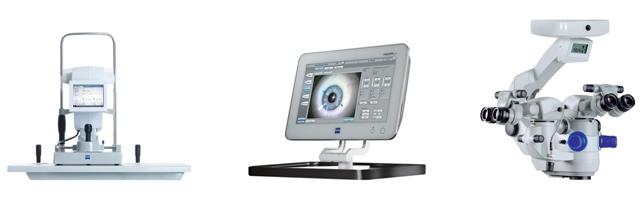
The ZEISS Cataract Suite brings together two gold standard technologies – the IOLMaster® 500 optical biometer and the OPMI LUMERA® 700 surgical microscope – with the innovative CALLISTO eye OR management system. The CALLISTO eye with its high level of precision assists cataract surgeons in the precise alignment of IOLs and is an integral part of the ZEISS Cataract Suite along with the OPMI LUMERA 700 microscope – which offers brilliant red reflex and unmatched clarity through its patented stereo coaxial illumination (SCI™) technology, and the IOLMaster 500 – which delivers a measurement success ratio of up to 20% higher than that of other optical biometers.
“High patient expectations and increasing workload due to aging demographics, coupled with declining reimbursements are driving the need for technologies that enable surgeons to perform complex procedures with greater confidence and to achieve optimal patient outcomes,” says Dr. Ludwin Monz, President and CEO of Carl Zeiss Meditec AG. “By bringing together the IOLMaster 500, OPMI LUMERA 700 and CALLISTO eye, the ZEISS Cataract Suite offers efficiency and precision at each crucial step: precise axial measurements, precise visualization and precise assistance to enable cataract surgeons to deliver expert outcomes.”
In addition to documenting surgeries in high definition, the CALLISTO eye works seamlessly with the OPMI LUMERA 700 providing graphical visual aids to assist in guiding precise incisions and LRIs (limbal relaxing incisions), capsulorhexis of optimal size and shape, and more accurate alignment of toric IOLs -- within ~3 degrees of intended target. These innovative visual aids can be viewed on the CALLISTO eye monitor screen and can be superimposed onto the live surgery image in the eyepieces of the OPMI LUMERA 700 microscope using the proprietary Integrated Data Injection System (IDIS). CALLISTO eye also provides easy access to patient information in the OR, and can be an auxiliary interface for controlling the microscope.
US release of new FORUM Viewer App
Carl Zeiss Meditec is also introducing at the ASCRS-ASOA the FORUM® Viewer App for iPads®, an extension to the company’s FORUM Eye Care Data Management system. The new FORUM software application extends FORUM Viewer to iPads allowing doctors convenient access to diagnostic patient data wherever and whenever they may want it. FORUM is a data management system for ophthalmology and optometry. FORUM allows data availability through central storage, increases workflow efficiency by providing data from multiple instruments and supports doctors in their clinical assessments.
US Clinical Trial of new Minimally Invasive, All-Femto Laser Vision Correction Method
Updates on the IDE clinical trial in the US for the ReLEx smile procedure for the correction of spherical myopia with the VisuMax® femtosecond laser will be presented by Jon Dishler, MD, the Medical Monitor for the US trial, during the ASCRS plenary session on Sunday afternoon April 21, 2013: Early Results from Clinical Trial on Femtosecond Laser Corneal Lenticule Extraction for Correction of Myopia.
New family of CIRRUS™ OCT products
Also on display during the 2013 ASCRS-ASOA at the ZEISS booth will be the new CIRRUS™ OCT family. The four new models – the CIRRUS 5000 and 500 and CIRRUS photo 800 and 600 – span the full spectrum of multi-modality imaging for glaucoma and retinal disease and addresses specific workflow and practice needs.
Attendees of the 2013 ASCRS-ASOA Symposium and Congress can experience ZEISS‘ state-of-the-art innovations and practice solutions at ZEISS booth #625 at the Moscone Center in San Francisco, April 20 to 23, 2013.