Oct 29 2012
Our master circadian clock resides in a small group of about 10'000 neurons in the brain, called the suprachiasmatic nucleus.
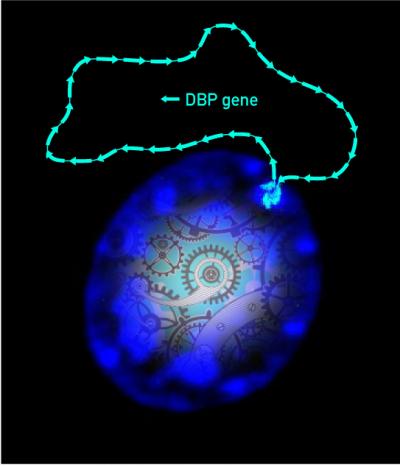 This is a picture of a living cell with fluorescent blue series of DBP gene copies...and stylized cogwheels. Credit: Markus Stratmann & Nicolas Roggli / University of Geneva
This is a picture of a living cell with fluorescent blue series of DBP gene copies...and stylized cogwheels. Credit: Markus Stratmann & Nicolas Roggli / University of Geneva
However, similar clocks are ticking in nearly all cells of the body, as demonstrated by the group of Ueli Schibler, professor at the Department of Molecular Biology of the University of Geneva, Switzerland. The molecular mechanisms of circadian clocks can thus be studied outside of the animals, in cultured cells.
A system to study gene regulation live in single cells
"Given the important role of the DBP protein in the regulation of detoxifying enzymes, we were interested in studying the molecular mechanisms underlying the rhythmic transcription of the DBP gene", points out the biologist, who is member of the NCCR Frontiers in Genetics. To do so, his team devised an elegant method to watch directly under the microscope how the clock's molecular "cogwheels" govern the activity rhythms of this gene in individual living cells. To this end, the scientists engineered a cell line with a piece of a chromosome exclusively composed of repeated DBP gene copies. They showed that the daily transcription of DBP is due to the rhythmic association of an essential clock component, the transcription factor BMAL1. "This is the first time a transcription factor binding to a circadian gene could be visualized in real time in single cells" explains Markus Stratmann, first author of the article.
The clock transcription factor must be sacrificed
To their surprise, the scientists found that the clock protein BMAL1 is destroyed while stimulating the expression of the DBP gene. By applying a variety of sophisticated imaging and biochemical techniques, they showed that the BMAL1 molecules bound to the DBP gene are degraded by an intracellular protein destruction machine, termed the proteasome.
Curiously, the chopping of the triggering protein BMAL1 is absolutely required for the efficient activation of the DBP gene. In other words, BMAL1 must die while embracing that gene in order to do its job. "In a sense, these transcription factors have the same cruel fate as males of the carnivorous insect Mantis. Sadly, Mantis females decapitate and then start eating their partners before the act of love is even completed" says Markus Stratmann.
At the moment, the biologists can only speculate about the broader impact of their findings. "We do not yet understand why the destruction of the BMAL1 protein is mandatory for the optimal functioning of the DBP gene" remarks Ueli Schibler. In fact, BMAL1 molecules regulate the daily activity of many other genes without getting killed while doing their work. The researchers noticed, however, that genes whose activity is not associated with the destruction of BMAL1 are expressed many hours later than the DBP gene. Their work thus offers a plausible explanation to the enigma of how one and the same transcription factor, BMAL1, can impose dramatically different daily cycles of gene expression.