Upon receiving a conditional approval from the US Food and Drug Administration (FDA) on behalf of the trial, Carl Zeiss Meditec has declared that its ReLEx smile procedure, which is implemented for the correction of myopia, will go through a US clinical trial.
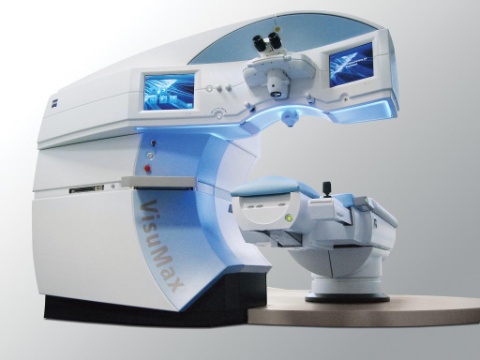 isuMax(R) Femtosecond Laser (Graphic: Business Wire)
isuMax(R) Femtosecond Laser (Graphic: Business Wire)
Carl Zeiss Meditec has formulated the new ReLEx smile technique that can be incorporated in refractive surgery. Carl Zeiss Meditec’s VisuMax Femtosecond Laser is a single system that integrates both precise lenticule extraction and femtosecond laser technology, which together will support the first-time minimally invasive laser vision correction procedure. The ReLEx smile method involves the production of a refractive lenticule in the intact cornea with the femtosecond laser, whereas the tissue is vaporized by the excimer laser used in LASIK procedures. The ReLEx method is then followed by the amputation of the lenticule by a small, 4mm or less incision. During this procedure, the patient can remain in the same place, with no need to move to an excimer laser.
The President and CEO of Carl Zeiss Meditec AG, Dr. Ludwin Monz says that the initiation of this clinical trial signifies the first milestone to success, besides benefiting the US surgeons with this procedure.
The VisuMax Laser Keratome has recently achieved US clearance for developing corneal flaps and for lamellar and corneal transplantations involving penetrating keratoplasty. VisuMax ReLEx smile is a single-step, first small incision, all-femto vision correction method. It had its worldwide launch during September 2011.
Conditional approval for carrying out the clinical trial was presented on April 20, 2012. Carl Zeiss Meditec anticipates enrolling patients for US clinical trial in another few months.