
Image Credit: Galyamin Sergej/Shutterstock.com
In 1921 Albert Einstein won the Nobel Prize for discovering the law which governs the photoelectric effect. Einstein put forward a revolutionary theory of light, showing that it behaves with the characteristics of particles as well as waves. This new understanding of photons and particle behaviour set off a cascade of discoveries which later merged into what is today known as quantum theory.
Light-Matter Interactions
The photoelectric effect is a process in which the energy carried in a photon is transmitted to an electron in the surface of a solid material. The electron absorbs the photon’s energy and is displaced from the surface of the material, creating a small electric current.
The photoelectric effect had been observed by physicists prior to Einstein’s work, but no one had been able to account for the fact that light below a certain frequency does not dislodge an electron from a surface of a material, irrespective of the intensity of the incident light beam.
An Unexplained Problem
In 1886, Heinrich Hertz was working on an experiment to generate electromagnetic radiation which used a high-voltage induction coil in order to generate a spark which would leap across a gap between two pieces of brass. As a result of having trouble seeing the spark, Hertz tried running his apparatus in a dark room and in doing so found that he had to make the gap much smaller. Hertz found that the spark lost strength when no visible light was striking the brass transmitter.
Intrigued by this observation, Hertz ran a number of experiments on the types and magnitude of light which had this effect by breaking down light into its various frequencies using a quartz crystal. Hertz found that ultraviolet wavelengths created the most powerful electrical currents but was not able to explain this phenomenon.
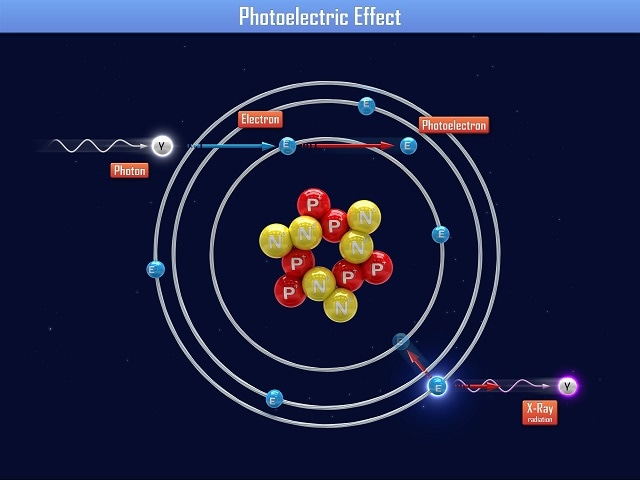
Image Credit: general-fmv/Shutterstock.com
In 1899, J.J. Thomson made the discovery that ultraviolet light could cause the brass to emit electrons and in 1902 Philipp Lenard discovered that increasing the intensity of light would cause greater numbers of electrons to be emitted from the surface of the material. To Lenard's surprise, increasing the intensity of the incident light beam had no effect on the level of energy of the emitted electrons. Lenard found that the only way to increase the electrons’ energy was to use light with a higher frequency, closed toward the ultraviolet end of the spectrum.
A Bright Spark
Until Einstein, classical physics considered light only as a wave phenomenon, and as a result of this established viewpoint, Lenard was not able to explain the outcome of his work with the photoelectric effect. Einstein offered an elegantly simple new interpretation of the results. Einstein hypothesized that light was composed of discreet quanta known as photons – the greater the frequency of light, the more energy those photons would have.
The photoelectric principles discovered by Einstein have been incorporated into the fabric of 20th-century technology, with video camera tubes and semiconductors relying on the movement of electrons stimulated by light. In addition, photovoltaic cells, commonly known as solar panels, rely heavily on the principle of electrons being emitted from material and entering a solid electrode, thereby converting radiant energy directly to electrical energy.
Sources and Further Reading
This article has been produced in association with the Pittcon Conference and Expo to celebrate the International Year of Light 2015.
For more information on how Pittcon are involved in IYL 2015, please visit www.pittcon.org