The cement industry faces a very interesting challenge in terms of quantitative analysis of clinker phases. Aside from traditional chemical analysis with XRF techniques, the role of XRD has been highly solicited for analyzing phases or minerals.
When it comes to controlling the kiln process and the quality of the end product, analyzing phases such as free lime in clinkers and limestone additions in cement by XRD, respectively, has been very useful.
Over the past decade, XRD has substituted traditional wet chemical or other methods in many cement laboratories. To address clinker phase analysis, a study was performed to introduce cost-effective, reliable, and rapid methods in real-time kiln conditions and examine the potential use of XRD as a substitute for microscopy or other indirect methods of calculation.
Instrument and Sample Preparation
For this study, a Thermo Scientific ARL 9900 Series was used. The instrument features a series of XRF fixed channels (monochromators), an XRF goniometer, and an XRD goniometer. This latter goniometer is integrated so that with the same tube conditions and under a vacuum, both XRD and XRF measurements can be done on the same sample.
A series of 30 clinker samples were collected over the production time. To obtain quantitative data for C3S, C2S, C3A and C4AF phases, microscopy measurements were done in parallel on the 30 samples.
A set of samples were chosen so that a reasonable dynamic range (working range) for concentrations of the four clinker phases could be obtained. Before being measured by the ARL 9900 Series Total Cement Analyzer, these clinker granules were ground and pressed into pellets.
During the normal microscopic observation of clinkers, the presence of a vitreous interstitial phase, which makes it difficult to differentiate the ferrite and the aluminum oxide, is typically the biggest challenge to overcome. This does not happen with the study samples since the clinker samples are cooled slowly, allowing for C3A crystallization.
On the other hand, the microscopically determined interstitial phase (alumina and ferrite) is always smaller than that which is calculated in Bogue formulæ, thanks to the assumption that all the Al2O3 and Fe2O3 contribute to the formation of C3A and C4AF, without consideration of the possibility that part of those oxides can form solid solutions with silicates.
It takes less than 4 minutes to complete the total analysis using the compact integrated XRD system for free lime and the four clinker phases. Peak intensities are used directly in this process without corrections.
Results & Discussion
Table 1 demonstrates the results of chemical analysis (total oxide concentration) obtained by the XRF aspect of the instrument. Using Bogue formulæ, these concentrations are used to calculate the equivalent concentrations of clinker phases:
%C3S = (%CaO - %CaO free) x 4.07 - (%SiO2 x 7.6 + %Fe2O3 x 1.43 + %Al2O3 x 6.72)
%C2S = %SiO2 x 2.87 - C3S x 0.75
%C4AF = %Fe2O3 x 3.04
%C3A = %Al2O3 x 2.65 - %Fe2O3 x 1.69
Table 1 displays the results of the clinker phases gathered using the compact XRD system combined in the ARL 9900 compared against the values obtained from microscopy and the Bogue calculations.
Table 1. XRF analysis of clinkers. Source: Thermo Fisher Scientific - Elemental Analyzers and Phase Analyzers
| Identification |
XRF analysis of oxides |
| Clinker sample |
CaO |
SiO2 |
Al2O3 |
Fe2O3 |
MgO |
SO3 |
Na2O |
K2O |
P2O5 |
TiO2 |
Mn2O3 |
Total |
| 1 |
66.62 |
23.73 |
5.16 |
3.17 |
0.75 |
0.65 |
0.14 |
0.73 |
0.36 |
0.29 |
0.02 |
101.6 |
| 2 |
66.36 |
21.77 |
4.99 |
3.2 |
0.75 |
0.84 |
0.14 |
1.01 |
0.36 |
0.29 |
0.02 |
99.73 |
| 3 |
66.64 |
23.56 |
5.22 |
3.41 |
0.77 |
0.53 |
0.14 |
0.52 |
0.36 |
0.3 |
0.03 |
101.45 |
| 4 |
66.57 |
22.52 |
5.13 |
3.34 |
0.75 |
0.66 |
0.14 |
0.87 |
0.36 |
0.3 |
0.03 |
100.65 |
| 5 |
66.47 |
23.86 |
5.38 |
3.69 |
0.76 |
0.45 |
0.13 |
0.42 |
0.36 |
0.31 |
0.03 |
101.83 |
| 6 |
66.56 |
23.15 |
5.08 |
3.21 |
0.75 |
0.6 |
0.14 |
0.7 |
0.35 |
0.3 |
0.03 |
100.85 |
| 7 |
66.55 |
23.66 |
5.33 |
3.47 |
0.75 |
0.56 |
0.14 |
0.62 |
0.36 |
0.3 |
0.02 |
101.74 |
| 8 |
66.51 |
23.37 |
5.14 |
3.55 |
0.75 |
0.62 |
0.14 |
0.73 |
0.36 |
0.3 |
0.03 |
101.49 |
| 9 |
66.73 |
23.58 |
5.24 |
3.25 |
0.75 |
0.53 |
0.14 |
0.53 |
0.35 |
0.31 |
0.02 |
101.41 |
| 10 |
66.66 |
23.11 |
5.1 |
3.25 |
0.75 |
0.72 |
0.14 |
0.68 |
0.36 |
0.3 |
0.03 |
101.07 |
| 11 |
66.64 |
23.33 |
5.13 |
3.25 |
0.75 |
0.59 |
0.14 |
0.61 |
0.36 |
0.3 |
0.03 |
101.11 |
| 12 |
66.71 |
22.91 |
5.15 |
3.35 |
0.75 |
0.5 |
0.13 |
0.57 |
0.36 |
0.29 |
0.02 |
100.72 |
| 13 |
66.62 |
22.82 |
5.19 |
3.43 |
0.75 |
0.57 |
0.14 |
0.72 |
0.36 |
0.3 |
0.02 |
100.89 |
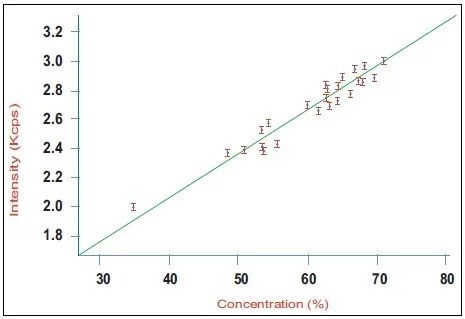
Figure 1. Calibration curve for alite (C3S) in a series of industrial clinker samples using the Integrated XRD system. Image Credit: Thermo Fisher Scientific - Elemental Analyzers and Phase Analyzers
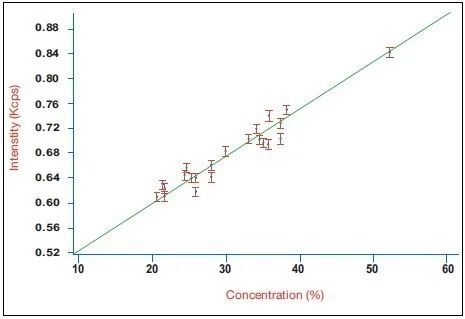
Figure 2. Calibration curve for belite (C2S) in a series of industrial clinker samples using the integrated XRD system. Image Credit: Thermo Fisher Scientific - Elemental Analyzers and Phase Analyzers
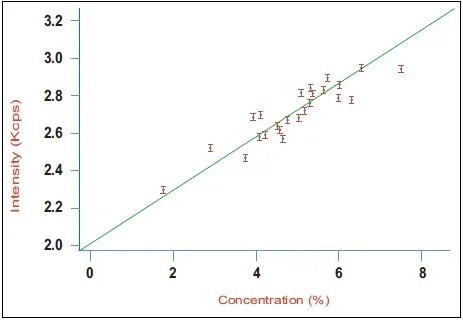
Figure 3. Calibration curve for aluminate (C3A) in a series of industrial clinker samples using the Integrated XRD system. Image Credit: Thermo Fisher Scientific - Elemental Analyzers and Phase Analyzers
Microscopy values and XRD results obtained with the ARL 9900 TCA have a strong correlation, while the Bogue data can create a number of errors in comparison to microscopy, as is shown in Figure 4. The very poor correlation between microscopy as a reference and Bogue calculation for C3S obtained using the data in Table 2 is displayed in Figure 5.
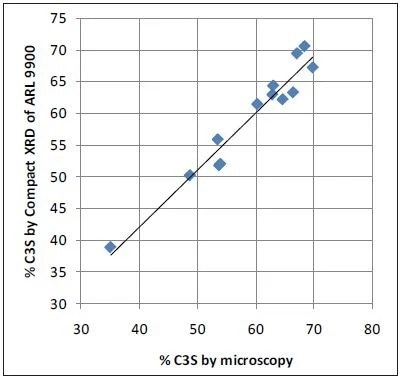
Figure 4. Microscopy vs Compact XRD for C3S: good correlation. Image Credit: Thermo Fisher Scientific - Elemental Analyzers and Phase Analyzers
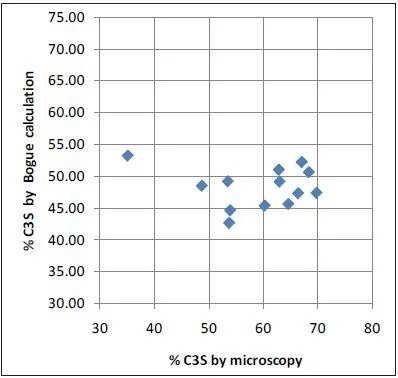
Figure 5. Microscopy vs Bogue XRD for C3S: no correlation. Image Credit: Thermo Fisher Scientific - Elemental Analyzers and Phase Analyzers
Table 2. Quantitative XRD analysis of free lime and clinker phases in comparison with microscopy and Bogue calculations. Source: Thermo Fisher Scientific - Elemental Analyzers and Phase Analyzers
| Identification |
Phases measured with integ. XRD of TCA |
Bogue's calculation |
Microscopy |
| Clinker sample |
CaO free |
C2S |
C3A |
C3S |
C4AF |
C2S |
C3A |
C3S |
C4AF |
C2S |
C3A |
C3S |
C4AF |
| 1 |
1.53 |
30.55 |
4.06 |
61.34 |
3.48 |
34.09 |
8.32 |
45.36 |
9.64 |
29.91 |
4.56 |
60.19 |
3.61 |
| 2 |
3.29 |
53.87 |
1.79 |
38.87 |
4.46 |
22.63 |
7.82 |
53.13 |
9.73 |
52.33 |
1.77 |
35.08 |
4.04 |
| 3 |
1.21 |
25.36 |
4.41 |
63.18 |
3.81 |
32.15 |
8.07 |
47.29 |
10.37 |
24.32 |
4.75 |
66.32 |
3.73 |
| 4 |
2.97 |
41.07 |
4.76 |
50.15 |
4.2 |
28.29 |
7.95 |
48.45 |
10.15 |
38.27 |
4.12 |
48.67 |
4.06 |
| 5 |
1.25 |
32.95 |
5.27 |
51.73 |
4.29 |
36.47 |
8.02 |
42.68 |
11.22 |
35.68 |
6 |
53.68 |
4.48 |
| 6 |
1.74 |
33.96 |
4.28 |
55.8 |
3.84 |
29.58 |
8.04 |
49.15 |
9.76 |
33.05 |
4.5 |
53.44 |
4.65 |
| 7 |
1.14 |
27.78 |
5.81 |
62.08 |
3.91 |
33.69 |
8.26 |
45.62 |
10.55 |
24.68 |
6.02 |
64.54 |
4.14 |
| 8 |
2.17 |
38.52 |
3.48 |
51.94 |
4.13 |
33.60 |
7.62 |
44.63 |
10.79 |
35.92 |
2.9 |
53.87 |
4.06 |
| 9 |
1.27 |
23.22 |
5.71 |
67.1 |
3.52 |
32.16 |
8.39 |
47.35 |
9.88 |
21.68 |
5.33 |
69.7 |
2.98 |
| 10 |
1.47 |
23.4 |
4.56 |
70.44 |
3.61 |
28.40 |
8.10 |
50.57 |
9.88 |
21.48 |
5.04 |
68.28 |
4.03 |
| 11 |
1.4 |
25.71 |
3.92 |
64.23 |
3.66 |
30.13 |
8.10 |
49.10 |
9.88 |
27.86 |
4.09 |
62.92 |
3.03 |
| 12 |
1.73 |
25.55 |
5.58 |
62.84 |
3.89 |
27.54 |
7.99 |
50.95 |
10.18 |
25.89 |
5.08 |
62.79 |
3.38 |
| 13 |
1.42 |
21.67 |
6.04 |
69.3 |
3.76 |
26.38 |
7.96 |
52.15 |
10.43 |
20.62 |
5.74 |
66.99 |
4.14 |
Conclusion
Within this case study, quantitative results were compared and obtained under three methods, including Bogue formulæ, microscopy and compact Integrated XRD using an ARL 9900 Series Total Cement Analyzer from a series of clinker samples.
It must be noted that the results from Bogue are not as anticipated. It is shown by the comparison between microscopy and quantitative XRD data that it is possible, in real process conditions for clinker phases analysis, to exploit the unique integrated compact XRD system of ARL 9900 Series.
Such XRD analysis can help predict the 3-day resistance to compression of the future cement obtained from these clinkers, as C3S phase provides resistance to cement, while other factors can influence 7-day resistance and 28-day resistance like fineness and particle size distribution, for instance.

This information has been sourced, reviewed and adapted from materials provided by Thermo Fisher Scientific - Elemental Analyzers and Phase Analyzers.
For more information on this source, please visit Thermo Fisher Scientific - Elemental Analyzers and Phase Analyzers.