A recent article in Light | Science & Applications introduced a novel imaging technique called "Spatiotemporally Resolved Synthetic-Scanning Photoacoustic Tracking (SRCPT)," which uses non-ionizing photoacoustic tomography (PAT) for deep tissue imaging. This method aims to enhance understanding of drug clearance pathways, particularly in critical metabolic organs such as the liver and kidneys.
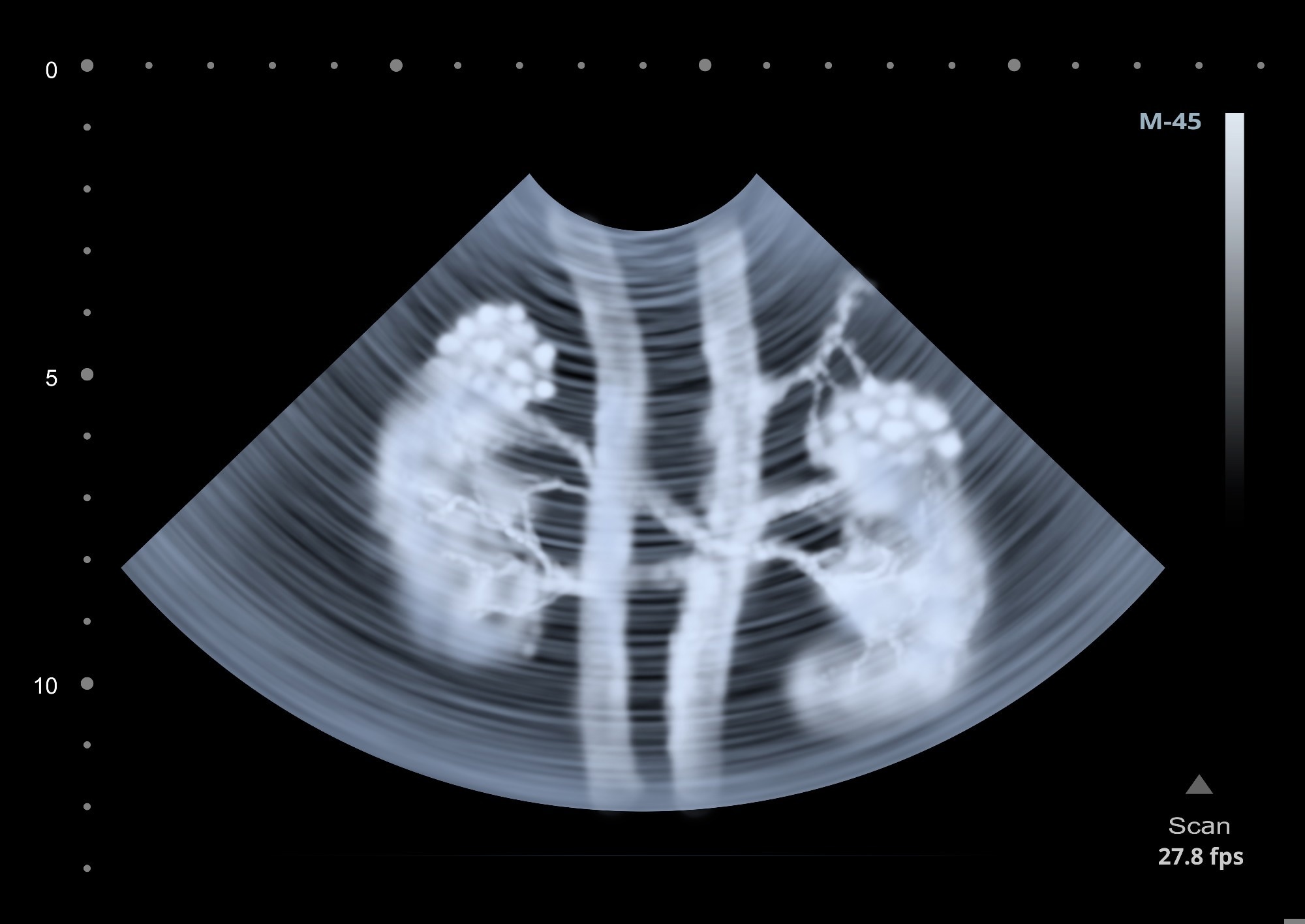
Image Credit: Alex Mit/Shutterstock.com
The researchers emphasized the importance of SRCPT for precision medicine, where targeted treatments are crucial. They also explored advanced algorithms to improve imaging resolution and accuracy in assessing drug clearance properties.
Advancement in Precision Medicine
Precision medicine requires a detailed understanding of drug clearance, especially in the liver and kidneys, which play essential roles in drug metabolism and excretion.
While traditional imaging methods such as positron emission tomography (PET) and single-photon emission computed tomography (SPECT) offer detailed imaging, they involve ionizing radiation, which limits their use for longitudinal studies. This has created a need for non-invasive, radiation-free imaging methods that provide high resolution and depth.
PAT is a promising alternative. It combines optical imaging with ultrasound, enabling deep tissue penetration and high-resolution imaging. By utilizing laser excitation and acoustic detection, this technique captures real-time images of biological structures and processes, making it ideal for studying drug clearance pathways.
Advanced algorithms, such as the Monte Carlo-corrected empirical mathematical model (MC-EMM), enhance PAT by addressing issues like laser fluence attenuation and improving both image quality and accuracy.
SRCPT: A Novel Method for Tissue Imaging
In this study, the authors developed and validated the SRCPT method, leveraging PAT to dynamically track drug clearance pathways in vivo. Combining advanced imaging techniques with mathematical modeling, they captured high-resolution images of drug distribution in the liver and kidneys of mice, focusing on the cancer drug mitoxantrone to analyze pharmacokinetics under normal and impaired liver and kidney functions.
The methodology involved creating a double-labeled probe, [68Ga]DFO-IRDye800CW, which has photoacoustic and PET properties. This dual functionality allows for detailed comparison and validation of SRCPT against traditional PET imaging. The study also introduced a frequency component selection-based synthetic aperture focusing technique (FCS-SAFT) that improves the resolution of three-dimensional (3D) imaging data.
The SRCPT setup included a wide-band laser to generate photoacoustic signals. This was paired with a full-ring ultrasonic transducer array for high-speed signal detection and a data acquisition system to record photoacoustic signals in real time.
The researchers used Monte Carlo simulations to accurately calculate light fluence within the tissues. They conducted experiments on eight-week-old male nude mice under conditions that simulated both normal and impaired organ functions.
This comprehensive setup enabled dynamic visualization of probe clearance properties and the extraction of key pharmacokinetic parameters, including peak time (Tmax), half-life (T1/2), average uptake rate (Uα), and average excretion rate (Eβ).
Key Findings and Insights
Results showed that mitoxantrone clearance primarily occurred through hepatic pathways, with limited metabolism in the kidneys. Under normal conditions, the drug quickly circulated through the liver, with a Tmax of 37.61 ± 9.52 seconds and a T1/2 of 128.53 ± 15.32 seconds. However, when liver function was impaired, the rate of hepatic metabolism dropped significantly, highlighting the liver’s crucial role in drug clearance.
The study reported a Tmax of 8.66 ± 2.14 seconds and a T1/2 of 10.02 ± 1.04 seconds in the kidneys, indicating slower clearance than in the liver. These results emphasized the liver's superior metabolic capacity compared to the kidneys. The average Uα in the liver was about 630 times higher than in the kidneys, reinforcing the liver’s primary role in metabolism.
The authors also highlighted the effectiveness of the FCS-SAFT algorithm, which improved imaging resolution. This algorithm separated high-frequency signals from blood vessels and low-frequency signals from tissue, enhancing the visibility of probe dynamics.
Additionally, the SRCPT method successfully tracked the clearance of immunoglobulin G (IgG) in mice with varying levels of acute kidney injury (AKI). It showed a significant increase in IgG deposition in the kidneys as AKI severity increased, demonstrating the technique’s potential for tracking biomacromolecule dynamics in kidney-related conditions.
Applications in Precision Medicine
This research has significant implications for precision medicine. The SRCPT technique can be used to monitor drug clearance pathways in various therapeutic settings, helping to create personalized treatment plans based on individual metabolic profiles. Its ability to visualize and measure drug dynamics in real time may enhance the effectiveness of drug formulations, leading to better therapeutic outcomes.
The findings can also support the development of new drug delivery systems and targeted therapies, especially in cases of impaired organ function. The SRCPT method shows potential for deepening understanding of pharmacokinetics and drug metabolism, which can improve therapeutic strategies in clinical practice.
Conclusion
In summary, the SRCPT method, combined with the MC-EMM algorithm, represents a significant advancement in biomedical imaging for studying drug clearance pathways in key metabolic organs. By providing high spatiotemporal resolution and real-time monitoring, this approach enhances understanding of pharmacokinetics and opens new opportunities in drug development and disease management.
The study highlighted the critical roles of the liver and kidneys in drug metabolism and emphasized SRCPT's potential in precision medicine. These insights could drive future advancements in pharmacokinetics and therapeutic optimization, ultimately improving patient care and outcomes in clinical practice.
More from AZoOptics: What Is the Role of Spectroscopy in Pharmacokinetics?
Journal Reference
Lv, J., et al. (2024). Dynamic synthetic-scanning photoacoustic tracking monitors hepatic and renal clearance pathway of exogeneous probes in vivo. Light Sci Appl. DOI: 10.1038/s41377-024-01644-6, https://www.nature.com/articles/s41377-024-01644-6
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.