Sep 17 2019
The presence of certain kinds of light causes many artificial and natural chemical systems to react and alter their characteristics. However, ordinary instruments cannot observe these reactions because they tend to take place too rapidly.
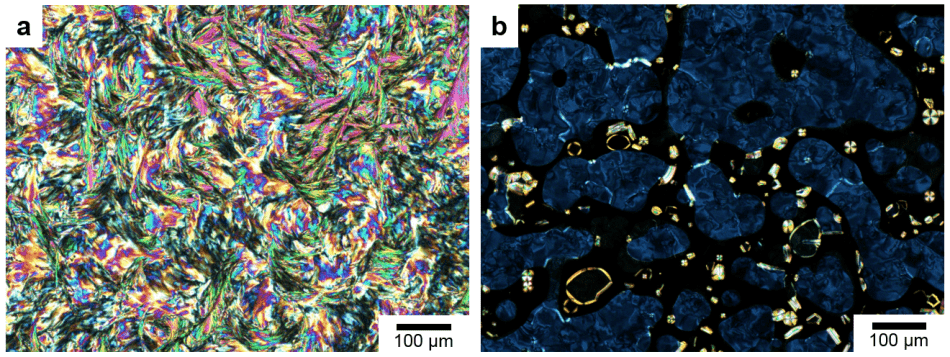 Polarized optical microscopy images of the molecules at 20 °C (A) and 51 °C (B). (Image credit: © 2019 Kato et al.)
Polarized optical microscopy images of the molecules at 20 °C (A) and 51 °C (B). (Image credit: © 2019 Kato et al.)
Now, for the first time, scientists have used an innovative method to observe these high-speed reactions. The technique reveals a new kind of reaction that may result in novel optical nanotechnology.
In chemistry, different things can be produced by exploiting molecules in many different ways. For instance, isomerization is a process that alters a molecular arrangement but does not touch constituent atoms.
Such a process can be seen in artificial systems such as specific kinds of chemical synthesis or in natural systems like the retina of the eye. In a majority of the cases, isomerization fundamentally renders a specific area of molecules either less ordered or more ordered.
Photoisomerization can be described as a kind of isomerization that is stimulated by light and occurs faster than the blink of an eye. In a study performed by Professor Takashi Kato from the Department of Chemistry and coworkers, liquid-crystal molecules of the chemical compound called azobenzene were subjected to certain frequencies of UV light.
The photoisomerization of one azobenzene molecule normally takes place on a timescale of quadrillionths of a second or hundreds of femtoseconds. That is equivalent to a billionth to a trillionth of the time it usually takes humans to blink their eyes. The scientists observed that the molecule subsequently activates molecular interactions in liquid crystals on a timescale of trillionths of a second or hundreds of picoseconds.
We have shown how to change the shape of azobenzene molecules from a straight rod shape to a slightly bent shape in a process triggered by photo-irradiation of UV light. This bending could translate to some mechanical or electronic function. The reaction propagates through neighboring molecules in the sample, meaning it is an extremely efficient process.
Professor Takashi Kato, Department of Chemistry and Biotechnology, School of Engineering, The University of Tokyo
While this kind of reaction does not occur separately, it does take place inside a soft matter sample whose function relies on the constituent molecules as well as their behaviors. Soft matter, in this case, can imply anything from flexible photographic sensors to an artificial muscle, or even things that have not been imagined yet.
Here, the significant fact is that the preliminary reaction, which normally occurs on a timescale of just hundreds of femtoseconds, triggers a reaction in the surrounding soft matter in about a timescale of hundred picoseconds, and it does this very efficiently.
“This is the fastest intermolecular motion ever observed within soft matter. In fact what we wanted to observe was so fast we had to use some very specialized methods to acquire data and to visualize what took place during these miniscule timeframes,” continued Kato. “This would not have been possible without some unique handmade spectral instruments made by my colleague Associate Professor Masaki Hada from the University of Tsukuba.”
The techniques are recognized as ultrafast time-resolved electron diffraction, which is similar to an X-ray and is how the reaction’s images were visualized, and also as ultrafast transient transmission spectroscopy, which provides a precise method to capture the makeup of a molecular sample.
It must be noted that both techniques are known as “ultrafast,” which further demonstrates that other techniques would not have been adequate to record data with the time resolution required by the scientists.
I have worked on ordered molecular assemblies such as self-assembling systems for more than 35 years as a chemist since I was a graduate student. This research advances the fundamental chemistry of photoresponsive molecules in soft matter as well as their ultrafast photomechanical applications.
Professor Takashi Kato, Department of Chemistry and Biotechnology, School of Engineering, The University of Tokyo
Kato continued, “It is a real privilege for myself and colleagues to work on this kind of project. We hope this may contribute to the design of molecular-based materials such as soft-body mechanisms and photo-functional materials.”