Jun 28 2017
For the first time in the world, Researchers, from the Institute for Molecular Science (IMS), Innovation Research Center for Fuel Cells, University of Electro-Communications, Research Center for Materials Science, Nagoya University, and JASRI (Japan Synchrotron Radiation Research Institute), have enhanced an ambient-pressure photoelectron spectroscopy instrument by using hard X-rays produced at SPring-8 and succeeded in photoelectron spectrometry under real atmospheric pressure. The journal Applied Physics Express has published a report on the achievements of these Researchers.
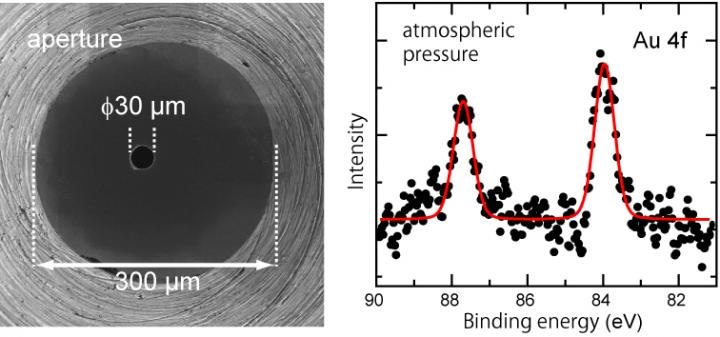 (left) This is a picture of a front cone, a circular cone-shaped spectrometer component, taken from above. The 30 µm aperture created at the tip is the port where photoelectrons enter the spectrometer. (right) The peaks represent the photoelectron spectroscopic signals of gold thin film detected under atmospheric pressure of air.
CREDIT: INSTITUTE FOR MOLECULAR SCIENCE
(left) This is a picture of a front cone, a circular cone-shaped spectrometer component, taken from above. The 30 µm aperture created at the tip is the port where photoelectrons enter the spectrometer. (right) The peaks represent the photoelectron spectroscopic signals of gold thin film detected under atmospheric pressure of air.
CREDIT: INSTITUTE FOR MOLECULAR SCIENCE
Standard photoelectron spectroscopy is capable of measuring only samples under high vacuum, while a number of catalytic reactions take place under atmospheric pressure. The inconsistency between the outcomes of the experiments under high vacuum and the real reaction mechanism under atmospheric pressure, "pressure gap," has been a problem. Over the recent years, this gap was filled by developing an apparatus known as, "ambient pressure photoelectron spectroscopy" which enables measurement under gas atmosphere. However, the upper-pressure limit of operation in a common pressure photoelectron spectrometer is about 5,000 Pa. It should be noted that even the apparatus with a presently reported world's highest performance has a limit of 15,000 Pa (approx. 0.15 atm), which is around 1/7 the atmospheric pressure (approx. 100,000 Pa). Thus, different groups from all over the world have been focusing on the development of photoelectron spectroscopy, which function under higher gas pressure.
An issue concerning measurement using ambient pressure photoelectron spectrometer refers to energy decay of the photoelectrons released from the sample exposed to light, which is because of the scattering caused by gas. This indeed restricts the upper-pressure of the measurement.
We made two improvements. First, we used hard X-rays that has higher energy compared to soft X-rays and boosted kinetic energy of the photoelectrons. Next, we created an extremely tiny aperture of 30 μm in diameter (figure left), which is a port that accepts photoelectrons into the spectrometer. This enabled to shorten the distance between the sample and the aperture, i.e. the distance of photoelectron traveling through gas has shortened.
Yasumasa Takagi, an Assistant Professor of IMS
Thus, the Researchers, for the very first time in the world, used gold thin film as a sample and succeeded in photoelectron spectroscopy under real atmospheric pressure.
Professor Toshihiko Yokoyama (IMS) believes in developing possibilities for future applications of the new photoelectron spectrometer. "Our apparatus achieved photoelectron spectroscopy under real atmospheric pressure, which greatly broadened its range of application. Reactions between solid and gas such as catalytic reactions and electrode reactions in fuel cells can be directly examined under atmospheric pressure. It can be also applied to biological samples that are fragile under high vacuum. In the future, photoelectron spectroscopy will be used for state analysis in various research areas."