Jan 25 2012
Researchers at the University of California, San Diego School of Medicine have created a new generation of fast-acting fluorescent dyes that optically highlight electrical activity in neuronal membranes. The work is published in this week’s online Early Edition of the Proceedings of the National Academy of Sciences.
The ability to visualize these small, fast-changing voltage differences between the interior and exterior of neurons – known as transmembrane potential – is considered a powerful method for deciphering how brain cells function and interact.
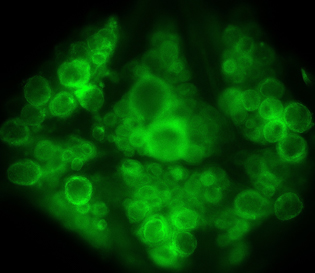 Leech neurons stained with voltage-sensitive dye.
Leech neurons stained with voltage-sensitive dye.
However, current monitoring methods fall short, said the study’s first author Evan W. Miller, a post-doctoral researcher in the lab of Roger Tsien, PhD, Howard Hughes Medical Institute investigator, UC San Diego professor of pharmacology, chemistry and biochemistry and 2008 Nobel Prize co-winner in chemistry for his work on green fluorescent protein.
“The most common method right now monitors the movement of calcium ions into the cell,” said Miller. “It provides some broad indication, but it’s an indirect measurement that misses activity we see when directly measuring voltage changes.”
The new method employs dyes that penetrate only the membrane of neurons, either in in vitro cells cultured with the dye or, for this study, taken up by neurons in a living leech model. When the dyed cells are exposed to light, neuronal firing causes the dye momentarily to glow more brightly, a flash that can be captured with a high-speed camera.
“One of the tradeoffs with using voltage-sensing dyes in the past is that when they were reasonably sensitive to voltage changes, they were slow compared to the actual physiological events,” said Miller. “The new dye gives big signals but is much faster and doesn’t perturb the neurons. We essentially see no lag time between the optical signal and electrodes (used to double-check neuronal activity).”
The new method provides a wider view of neuronal activity, said Miller. More importantly, it makes it possible for neuroscientists to do accurate, single trial experiments. “Right now, you have to repeat experiments with cells, and then average the results, which is physiologically less relevant and meaningful.”
For Tsien, the new dyes address a career-long challenge.
“These results are the first demonstration of a new mechanism to sense membrane voltage, which is particularly satisfying to me because this was the first problem I started working on as a graduate student in 1972, with little success back then,” said Tsien. “Later, we devised indirect solutions such as calcium imaging or dyes that gave big but slow responses to voltage. These techniques have been very useful in other areas of biology or in drug screening, but didn’t properly solve the original problem. I think we are finally on the right track, four decades later.”
Funding for this research came, in part, from the Howard Hughes Medical Institute, the National Institutes of Health, including the National Institute of Neurological Disorders and Stroke and the National Institute of Biomedical Imaging and Bioengineering.
Co-authors are John Y. Lin, Department of Pharmacology, UC San Diego; E. Paxon Frady, Neurosciences Graduate Group, UC San Diego; Paul A. Steinbach, Department of Pharmacology, UC San Diego and Howard Hughes Medical Institute; William B. Kristan, Jr., Division of Biological Sciences, UC San Diego.