Fluorescence-based flow cytometers have found application across a variety of biological disciplines. They are capable of rapidly and accurately quantifying cells and cellular components and as one of the most important components of these systems, flow cytometry filters need to be specially designed in order to maximize signal-to-noise ratios, as well as minimizing crosstalk between fluorophores.
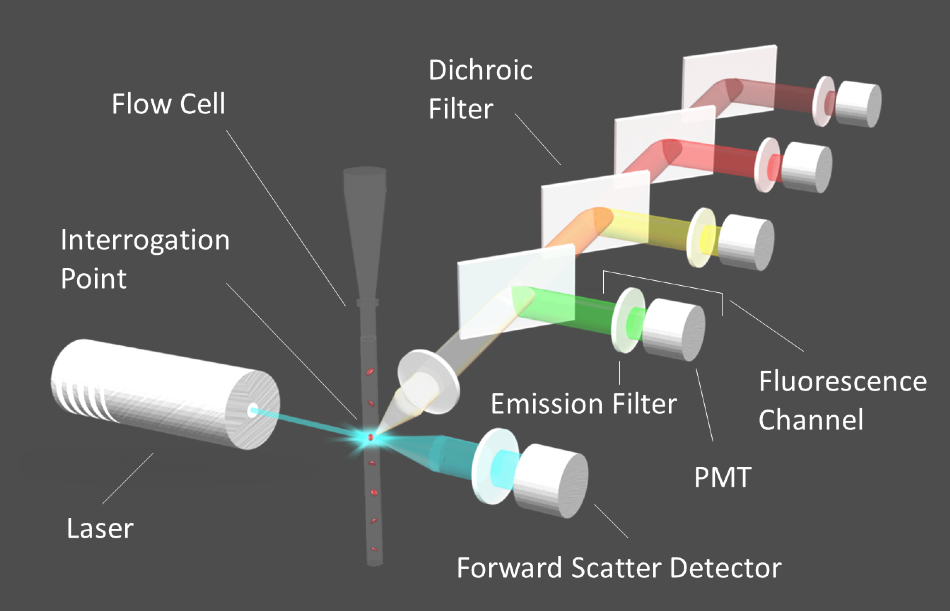
Figure 1. Diagram of a flow cytometer. Image Credit: Alluxa
How Flow Cytometers Work
Flow cytometers are fluorescence-based systems that are used to quantify and sort cells that have been tagged with one or more fluorophores. To begin the process, individual cells are labeled with fluorescent tags. They are then suspended in a physiological buffer known as a sheath fluid. Once suspended, they are injected into the fluidics system of the flow cytometer.
The suspended cells are arranged in a single-file row by means of hydrodynamic focusing once they are inside the flow cell of the fluidics system. Interrogation points are placed at intervals along the flow tube, where a laser beam excites the fluorophores in the sample as the line of cells moves through the flow tube. The fluorescence emission signals from each fluorophore are then isolated by their corresponding emission filters, to then be directed to the appropriate detector through a series of dichroic filters (Figure 1).
Photomultiplier tube (PMT) detectors that work to convert light into electric currents make up part of a flow cytometer. The electric current generated by the PMT is digitized for analysis. As cells flow past the interrogation points, an electric pulse is generated, which corresponds to the intensity of the signal at each point in the cell's path as it passes the laser beam.
The signal intensity returns to baseline when the cell has flowed completely past the beam, until the next cell reaches the interrogation point. After this, the pulses are quantified to provide accurate cell counts or information on specific cellular components. In cell sorters, cells are directed to vials according to which fluorophores are detected within them.
Flow Cytometry Filters
Flow cytometers utilize fluorescence emission filters and dichroic filters. These filters are specially designed to isolate the relatively weak emission signals of the target fluorophore for each channel, while also blocking the signals of neighboring fluorophores (Figure 2).
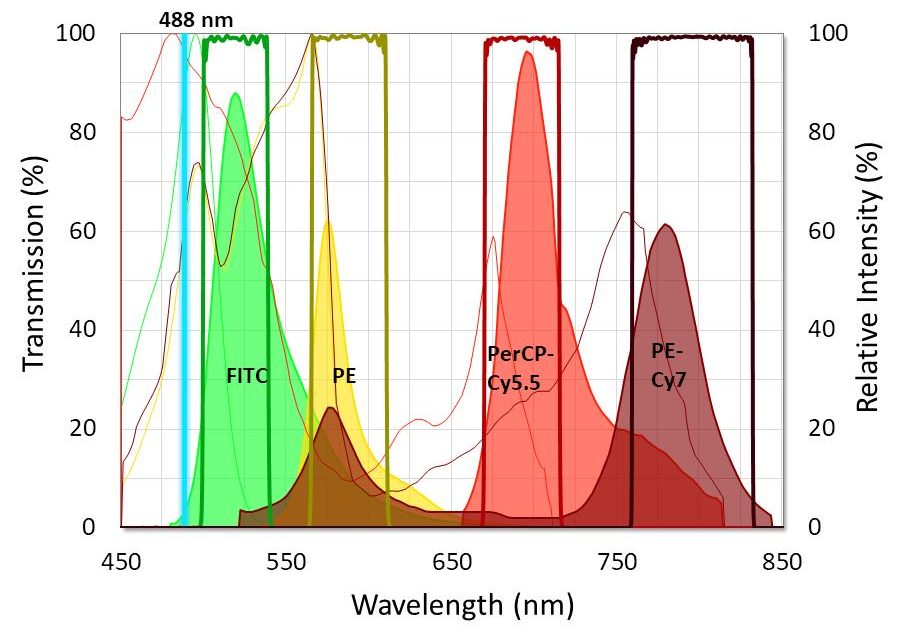
Figure 2. Graph showing the ideal placement of flow cytometry filters used with four standard fluorophores. Fluorophore emission intensity is shown after excitation with a 488 nm laser. Image Credit: Alluxa
To capture emission peaks of every fluorophore, it is essential that flow cytometry emission filters are designed with passbands that have high transmission and low ripple. It is also important that emission filters are built to have very steep edges and relatively narrow bandwidths. This is so they are able to block the majority of the overlap between fluorophore emissions. Wide-range, out-of-band blocking should extend beyond OD6 (-60 dB or 0.0001% transmission), especially at the excitation laser line.
So that dichroic filters for flow cytometry can direct the target fluorescence emission signals to the right detector, they have to be designed with steep edges and minimal polarization splitting. They should also have a surface flatness specification suitable for use with lasers to ensure that the spot size distortion and beam deviation, which is caused by reflected wavefront error (RWE), is effectively minimized.
In addition to this, because emission light will be transmitted through several filters before reaching the detector, all flow cytometry filters should be specifically designed to produce minimal transmitted wavefront error (TWE). This is so that they can prevent distortion of the signal. The laser damage threshold (LDT) should also be high in all of these filters used with fluorescence-based flow cytometry.
Cytometry Filters by Alluxa
An innovative SIRRUS plasma deposition process hard-coats all of Alluxa’s flow cytometry filters, which lets Alluxa produce high-performance laser clean-up, emission, and dichroic filters time and time again. These filters boast steep edges, a typical transmission of over 95%, precision wavelength control, low TWE, and a high LDT.
Dichroic beamsplitters for flow cytometry from Alluxa are also built to achieve surface flatness that is 2λ P-V per inch or better, depending on the specific requirements of the user’s flow cytometer.
Alluxa’s experienced engineers work closely with users to design the optimal flow cytometry filters for their systems, based on their fluorophore selections, excitation lasers, and system configuration.

This information has been sourced, reviewed and adapted from materials provided by Alluxa.
For more information on this source, please visit Alluxa.